Áron Szabó and his colleagues from the Institute of Genetics of the ELKH Biological Research Centre (BRC) conducted a study on glial degradation pathways in a model organism, the fruit fly Drosophila melanogaster in the laboratory led by Gábor Juhász. The researchers discovered that certain proteins of the autophagy pathway, the machinery responsible for the lysosomal degradation of intracellular material, regulate the degradation of axonal debris produced in abnormal circumstances, e.g., brain injury, neurodegenerative disease, or stroke. This process is known as LC3-associated phagocytosis (LAP). Since LAP is already known to be involved in debris clearance in astrocytes in the mammalian brain, it could also serve as a potential therapeutic target in the future. The study presenting the results was published in Nature Communications.
Our brains are in a constant state of change, not only cognitively but also physically, with important components constantly being constructed and broken down. In abnormal circumstances, this balance can be shifted for longer or shorter periods of time, whether in brain injury, neurodegenerative disease, or in the case of stroke or infection. One role of glial cells is to keep the brain clean under both normal and pathological conditions. Much is known about how glial cells recognize the material or microbes to be degraded, but there is less underlying research about how these are degraded in the lysosomes of glia, one of the digestive centers of the cell.
The researchers studied glial degradation pathways in a model organism, the fruit fly Drosophila melanogaster. They have discovered that certain proteins of the autophagy pathway, the machinery responsible for the lysosomal degradation of intracellular material, regulate the phagocytic degradation of axonal debris produced during injury. This is interesting because phagocytosis takes up and breaks down extracellular material, unlike autophagy. This process, known as LC3-associated phagocytosis (LAP), was originally studied in macrophages, but only began to be characterized in the nervous system in parallel with their research. Their experiments were performed by transecting a nerve running through the fruit fly wing, a simple, reproducible and easily visualized model of traumatic injury. By manipulating glia and neurons separately, they showed that the functions of autophagy-specific genes already known to contribute to LAP and LAP-specific genes are required for normal debris clearance by glia. The corresponding proteins are also found on vesicles containing phagocytosed axon fragments.
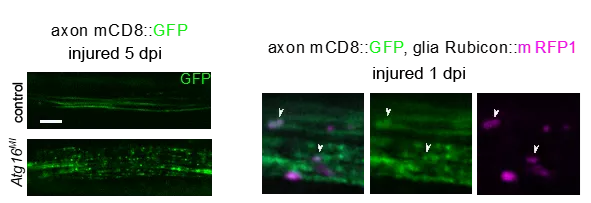
Too little or too much phagocytic activity in either macrophages or glial cells can be detrimental to vital functions. The absence of LAP after traumatic brain injury led to increased mortality in the long term, demonstrating the importance of debris degradation by LAP. One explanation for such lethal effects may be chronic inflammation, which can also lead to autoimmunity when macrophages are deficient in LAP. The enhancement of LAP through the overexpression of the Rubicon protein yielded faster clearance in the damaged nerve, which is a prerequisite for recovery. Since LAP is already known to be involved in debris clearance in astrocytes in the mammalian brain, this process could also serve as a potential therapeutic target in the future.